Mercury distillation
2009-08-26 20:30 by Ian
The distillation was carried out in 3 batches of 7 pounds each.
A simple distillation apparatus was assembled with PTFE tape forming the seals for the glassware. The coolant chiller was used in a closed-coolant-loop to cool the condenser. The recovery flask was immersed in an azeotropic mixture of methanol-water over dry ice for the purpose of freezing the purified mercury. This was purely a safety measure to reduce the release of mercury vapor upon disconnection of the recovery flask.
The vacuum adapter outlet was attached to a polyvinyl hose with the free-end immersed in a water bath to capture and condense any remaining mercury vapor while allowing excess pressure in the apparatus to escape.
After being isolated from the atmosphere, the entire distillation apparatus was flushed with CO2.
Apparatus was checked for leaks and flaws by two sets of eyes that have at least 7-semesters of chemistry lab between them. Then it was noted that mild negative pressure in the apparatus drew water up the polyvinyl tubing that did not descend, indicating no leaks.

238mL (7 pounds) of impure mercury was loaded into a dual-neck heavy-walled flat-bottom boiling flask. DOT3 brake fluid was used to form a thermal seal between the boiling flask and the hotplate. Heat was applied, and the mercury allowed to come to a boil at about 360C.
Distillation was allowed to proceed until dryness. Heat was allowed to rise to 380C to allow remaining vapor to condense and solidify in the recovery flask. Heat was lowered to 300C as the condenser was carefully agitated to dislodge large beads. Following this, the recovery flask removed. A storage bottle was flushed with CO2 and the recovery flask inverted to allow the melting mercury to drip into the storage bottle.
Update 08.28.2009
Shit. I baked my hotplate to death.
[sigh] More repairs…
The apparatus remained sealed off from atmosphere, and functioned flawlessly. Water flowed into the recovery flask and covered the distilled mercury. This continued until the inside and outside temperature reached equilibrium, and pressure was insufficient to draw additional water into the flask. No product was lost.
Distillation ceased in the first hour of 08.28.2009 after running for approximately 3 hours. Client will be shipped product that was successfully distilled and will have to wait for the remainder until hotplate can be diagnosed and repaired.
After examination of the mercury remaining in the boiling flask, the following observations were made:
As the concentration of the impurities increased (because mercury was leaving the flask, and impurities were not) the carrying capacity of the volume of remaining impure mercury was exceeded, causing a precipitate to form and float to the surface. The bulk of this were oxides of lead.
Most of the remainder was a dry substance that looked frighteningly like the sand from the sand bath. [How the hell did that get into the flask?!?]
After disassembly of the sand bath and close examination of the entire boiling flask, it was determined that this substance must have been solvated in the mercury to begin with. This hypothesis was strengthened by noting that the apparatus still held a pressure that was applied while watching the water level in the polyvinyl bleed tube.
LuLz were had salted with 12.5% relief. Work will continue after the hotplate is repaired or replaced.
Update 08.28.2009:
Hotplate diagnosed. The fuse melted. Not blew. The glass turned to liquid and having lost its rigidity, the fuse failed. A temporary repair was performed and the hotplate is once again operational. Distillation will be resumed tomorrow morning.
Update 09.09.2009:
Still waiting on the photos from my friend. But I did complete the distillation. And, my God… What came out of the source material shocked me. Please keep in mind: This was the same batch of mercury that I filtered in my earlier blog entry. Moreover, all of the glassware depicted below was clean when this operation began.
Here are a few shots of the boiling flask. The crud inside looks like fine dirt. I suspect that it’s copper, lead, and solvated dirt. The walls of the flask are coated with a course layer of what appears to be a lead-mercury amalgam. There was about 750mL of mercury that passed through this flask, 520mL of which made it through the distillation path, was collected and shipped to the client.

Here is a picture with the camera lens pressed to the glass wall of the boiling flask, to try to capture the inside. Please forgive the haze.

Here is the mercury that did not make it through the distillation path. This was emptied from the boiling flask, through a filter. This mercury is filthy all the way through.

It was amazing to see how much garbage can collect in otherwise excellent-looking mercury! I was in a hurry to get the product shipped, so I didn’t take any pictures of the distilled product. But it looked little different from the filtered stuff.
Update 29.06.2010:
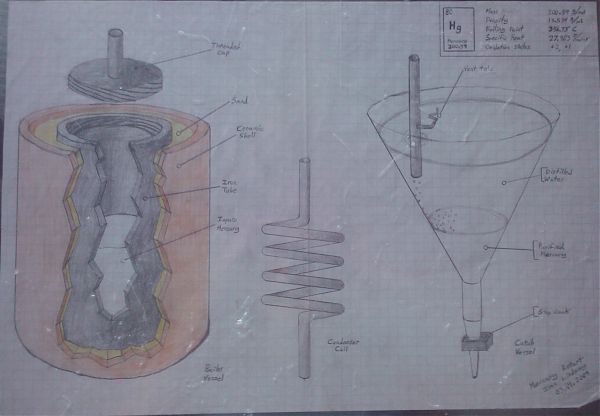
I drew this more than a year ago, but have just gotten around to photographing it and poasting it here. This is a mercury retort I devised.
Previous: Liquid metal
Next: An accessible introduction to PHP optimization